CODE-MI is a multi-centre stepped wedge, cluster randomized trial with an overall objective of evaluating the impact of using the female-specific 99th percentile cut-point for high-sensitivity cardiac troponin (hs-cTn), compared to the overall 99th percentile cut-point, on the diagnosis, treatment and outcomes of women presenting to the emergency department with cardiac chest pain.
Study Videos:
CODE-MI Overview: Dr. Karin Humphries
CODE-MI, ED Perspective: Dr. Jim Christenson
Study Objectives
To examine the impact of using a lower female-specific hs-cTn cut-point on:
- Diagnostic and therapeutic strategies
- Prognosis: all-cause mortality, readmission for non-fatal myocardial infarction, incident heart failure or emergent/urgent coronary revascularization at one year following presentation to ED department
- Costs of diagnostic testing and treatment
Study Design and Timeline
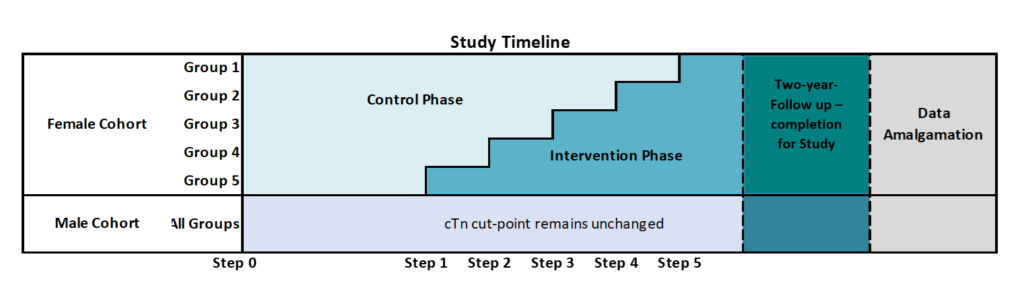
- Stepped wedge, cluster randomized trial, with clusters (hospitals) crossing over sequentially, in random order, from control to intervention phase
- Control Phase: Standard of care, with the use of single, overall hs-cTn cut-point in both sexes
- Intervention Phase: Introduction of female hs-cTn cut-point
- Initiation of randomization date: January 2020
- Approximate study end date: March 2025
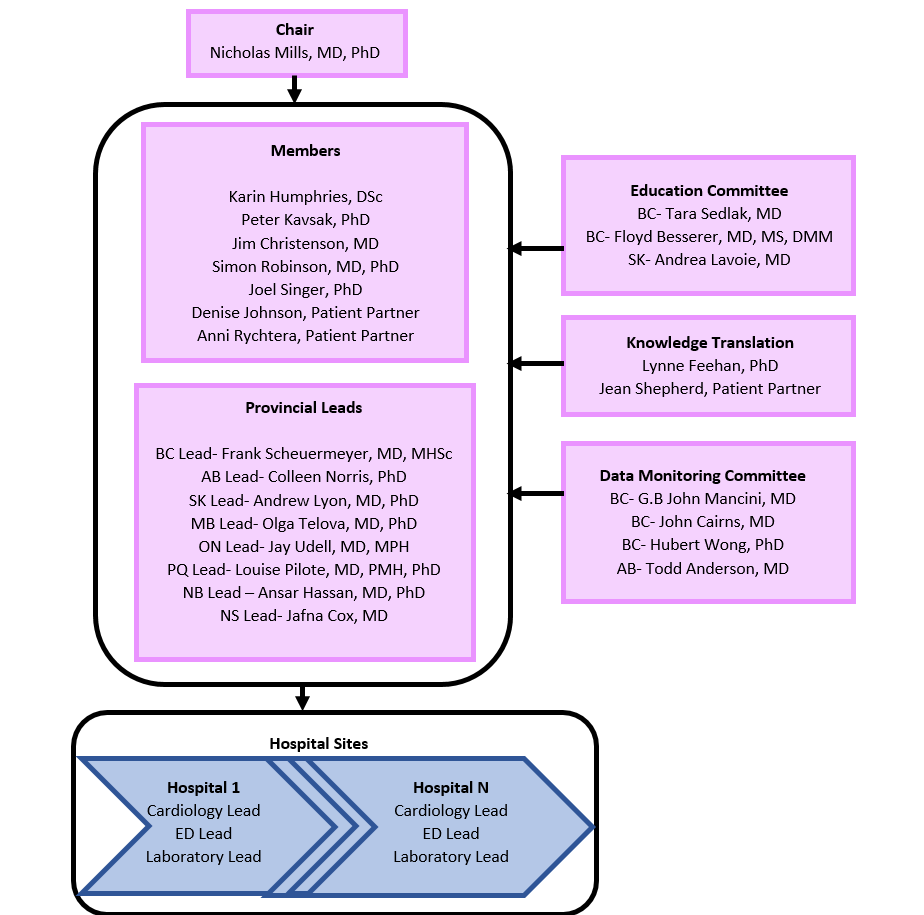
Study Organization
26 Participating Sites across Canada
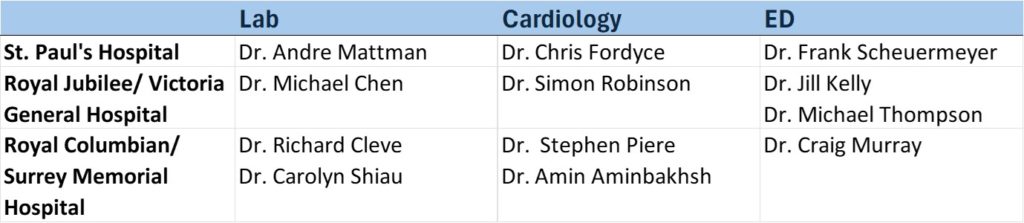
Participating Sites and Leads
British Columbia – Provincial Lead: Dr. Frank Scheuermeyer
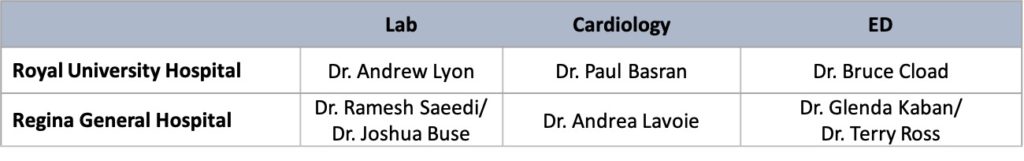
Saskatchewan – Provincial Lead: Dr. Andrew Lyon
Manitoba – (Control Site)

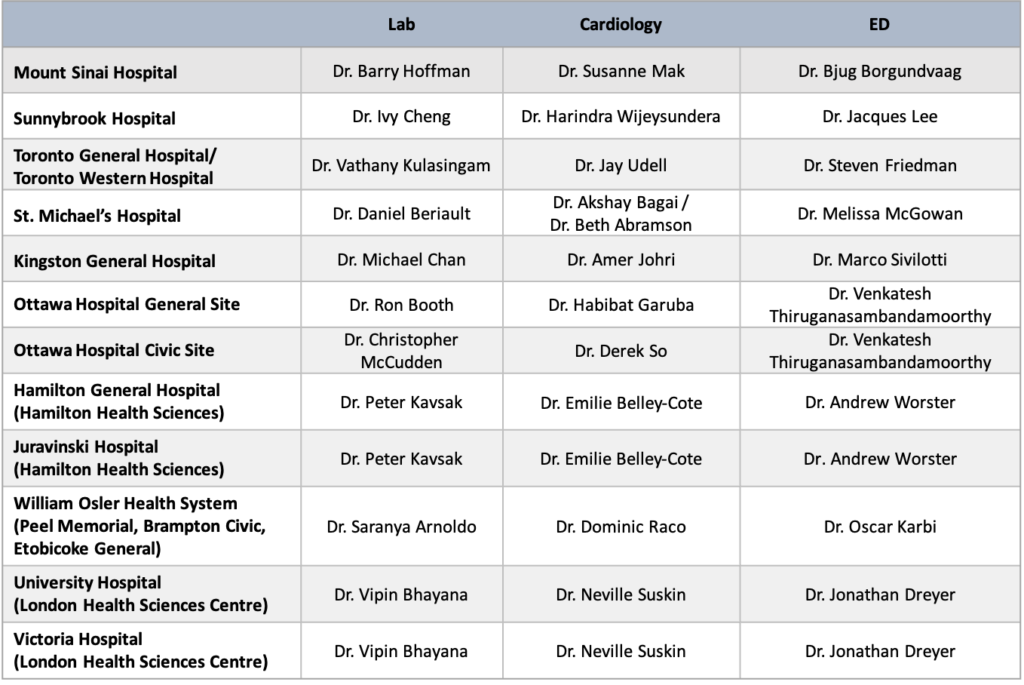
Ontario – Provincial Lead: Dr. Jay Udell
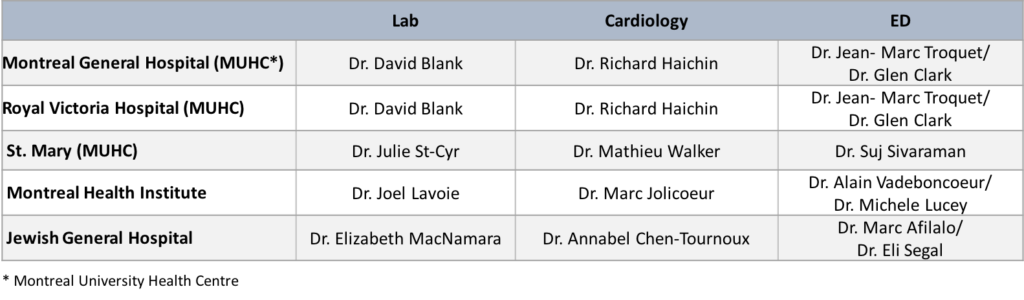
Quebec – Provincial Lead: Dr. Louise Pilote
New Brunswick – Provincial Lead: Dr. Ansar Hassan

Nova Scotia – Provincial Lead: Dr. Jafna Cox

